MycoTOOL Test Performance Data for Release Testing
Acceptance
- In 2009, the European Medicines Agency (EMA) accepted release testing with the MycoTOOL Test for a pharmaceutical product from Roche.
In 2011 the Ministry of Health, Labour and Welfare of Japan (MHLW) accepted release testing with the MycoTOOL test for a pharmaceutical product. Meanwhile Mycoplasma testing with the MycoTOOL Test is accepted by the FDA for different pharmaceutical products.
Alternative Validation Data
The European Pharmacopoeia 2.6.7 Guidelines* allow using published validation data instead of repeating user validation.
"...Where commercial kits are used for part or all of the analytical procedure, documented validation points already covered by the kit manufacturer can replace validation by the user. Nevertheless, the performance of the kit with respect to its intended use has to be demonstrated by the user..."
*E.P. 2.6.7 Guidelines, Chapter "Validation of NAT detection of Mycoplasmas"
The complete validation program with different validation approaches is published in a peer reviewed journal: Deutschmann SM, et al., "Validation of a NAT-based Mycoplasma assay according European Pharmacopoeia", Biologicals 38 (2010), pp. 238-248.
Refer to this publication to reduce your validation workload and complement your validation data.
Sensitivity
The Mycoplasma touch-down PCR was originally developed by Genentech in 2000 - 2003, and published in 2004:
Eldering JA, et al., "Development of a PCR method for mycoplasma testing of Chinese hamster ovary cell cultures used in the manufacture of recombinant therapeutic proteins", Biologicals 32 (2004), 183-193.
This "homebrew" method was transferred to Roche and an initial validation study was performed using reagents from several different vendors. The limit of detection was analyzed for the Mollicute species proposed in the European Pharmacopoeia Guidelines, Chapter 2.6.7:A. laidlawii, M. hominisM. pneumoniae, M. arginini, M. hyorhinis
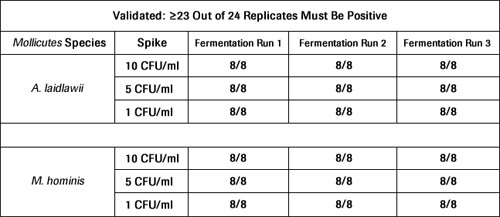
M. salivarium, M. fermentans, M. orale, S. citriAll dilutions where tested on a 95% confidence level as required. At least 23 out of 24 replicates had to be positive.
After the MycoTOOL Test was developed, the most difficult targets were retested in a subsequent validation approach (see Table 1). No UV irradiation of the reagents is needed to perform the validation with the MycoTOOL Kit. A comparison of the Roche "homebrew" data set with the MycoTOOL Kit data set clearly supports the superior quality of the purified Roche reagents.
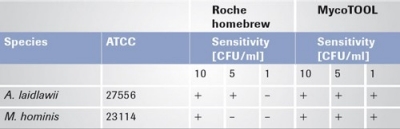
Tab. 1: Comparison of the sensitivity of the Roche homebrew test with the MycoTOOL Test.
Speed
Cellular samples as well as noncellular samples can be tested in less than 5 hours.
Security
Lysis and Inhibition Control of Matrix using Parallel GAPDH PCR
The risk of obtaining false negatives is of particular importance, since undetected contamination can have negative health consequences.
If the internal control is limited to using a plasmid, the sample preparation remains uncontrolled and a false negative result may not be detected. For this reason, it is important to monitor the efficiency of the sample preparation using a parallel matrix-specific PCR.
False negatives can potentially be caused by inefficient cell lysis, since mycoplasmas also survive within the cells. GAPDH PCR using a dilution of the matrix avoids that possibility since the result is positive only if the lysis of the matrix is fully efficient (see Fig. 2).
Further matrix inhibition or lack of sensitivity that is a function of the sample preparation reagents is monitored by undiluted GAPDH PCR.If amplification of the GAPDH gene is not appropriate for your application, Custom Biotech can adapt the MycoTOOL Test to your requirements; primers for other housekeeping genes are fully tested.
Carryover Contamination
The MycoTOOL Test uses dUTP instead of dTTP for incorporation into the PCR product. When a dUTP-containing amplification product contaminates the test workflow, it will be digested using the enzyme uracil-DNA glycosylase (UNG) instead of being reamplified in the subsequent PCR reaction. This UNG reagent is already included in the master mix.
A mycoplasma sequence with a deletion is used to code for the positive control. When the amplification product of this plasmid contaminates the PCR, it can easily be differentiated from a real mycoplasma contamination by its shorter length.
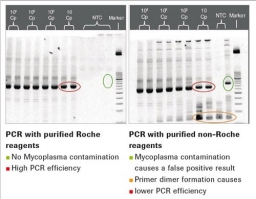
Fig. 3: Comparison of PCR with Myco TOOL oligos (left) and oligos from an oligo house (right). The circles mark nonspecific PCR artifacts.
Purified Reagents: Lot-to-Lot Consistency
False positives are prevented by using nucleic acid-free reagents, tested according to current quality control procedures at Roche. Figure 3 below compares a PCR with oligos from an oligo house (HPLC-purified) and a PCR with Roche nucleic acid-depleted oligos.
Change Control Notification
Design and production for the MycoTOOL Test are controlled by the Change Control Notification procedures of the Roche Quality Control system, ensuring that the product will retain its proven high quality.
Versatility
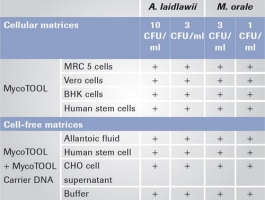
Mycoplasma release testing with the MycoTOOL Test is approved for cell lines growing in protein-rich media and at high-cellular densities.
For each of these difficult cell lines, the validation passes the acceptance criteria of 1 CFU/ml (see Tab 2).